The Science Behind Carbonated Drinks
Introduction
Carbonated drinks are an essential part of modern life. From sodas and sparkling waters to beers and energy drinks, they all share one thing in common: effervescence. But what is it about these fizzy beverages that make them so universally loved? The answer lies in the fascinating science of carbonation—a process that is not only responsible for the bubbles but also plays a crucial role in how these drinks taste and feel. In this article, we will delve into the chemistry behind carbonation, its impact on flavor and texture, and how it all comes together to create the refreshing experience we crave in a carbonated beverage.
What is Carbonation?
Carbonation is the process of dissolving carbon dioxide (CO2) gas into a liquid, creating the signature bubbles that we associate with fizzy drinks. This is typically done under high pressure, which allows the CO2 to dissolve into the liquid more efficiently. When the pressure is released (such as when opening a bottle), the dissolved gas forms bubbles and escapes, creating the fizzy sensation.
A refreshing glass of carbonated beverage made with CO2 canister
The process of carbonation has been practiced for centuries, starting with the creation of carbonated water in the 18th century. Since then, carbonation has evolved to become a key feature in many types of drinks, including soft drinks, sparkling waters, alcoholic beverages, and more.Discover our CO2 canisters for carbonating drinks.
The Chemistry Behind Carbonation
Understanding the science behind carbonation requires a basic knowledge of gas solubility. At its core, carbonation is a result of CO2 dissolving in water, and this process is influenced by both temperature and pressure. The chemical reaction occurs as follows:
CO₂ (gas) + H₂O (liquid) ⇌ H₂CO₃ (carbonic acid)
When carbon dioxide is dissolved in water, it reacts with water molecules to form carbonic acid (H₂CO₃). This is a reversible reaction, which means that carbonic acid can break back down into carbon dioxide and water when the pressure is reduced. This creates a dynamic equilibrium between the dissolved CO₂, carbonic acid, and the liquid.
Key Insights on Solubility:
Pressure: Higher pressure increases the solubility of CO2 in the liquid, allowing more CO2 to dissolve, which results in more bubbles once the pressure is released.
Temperature: Cold temperatures also help to retain the CO2 in the liquid. That’s why carbonated drinks are always more fizzy when served chilled. Warmer liquids have lower solubility for CO2, causing the gas to escape more quickly when opened.
The presence of carbonic acid in carbonated drinks contributes to their slightly acidic taste. This mild acidity is one of the factors that enhance the refreshing qualities of these beverages.
How Carbonation Affects the Taste and Texture of Drinks
One of the most significant effects of carbonation is how it impacts the taste and texture of drinks. The bubbles produced by dissolved CO2 are more than just a visual and sensory element—they change the way we perceive flavors.
Impact on Flavor
Carbonation acts as a flavor enhancer in many ways:
Balancing sweetness: The mild acidity of carbonic acid can counterbalance the sweetness in sodas, making them taste less sugary and more refreshing.
Enhancing flavors: The presence of CO2 can stimulate the taste buds more effectively, making flavors seem more vibrant and intense. This is one reason why people often describe carbonated drinks as having a sharper taste.
Mouthfeel: The bubbles themselves contribute to the texture of the drink. When you take a sip, the bubbles create a tingling sensation on your tongue, which adds an extra layer of texture. This bubbly mouthfeel is what gives carbonated drinks their signature "crispness" and makes them feel lighter and more refreshing than still beverages.
Effervescence and Texture
The effervescence of a drink contributes to its overall texture, which plays a critical role in our enjoyment. The carbonic acid not only adds acidity to the drink but also influences its body and feel:
Carbonation and lightness: The presence of bubbles makes carbonated drinks feel less heavy, which is why they are often preferred during meals or as a thirst-quencher.
Cleansing the palate: The effervescent bubbles help to refresh the palate, making carbonated drinks ideal for cleansing the taste between bites of food. This is why fizzy drinks are often paired with rich, greasy foods—they provide a contrast that enhances the overall eating experience.
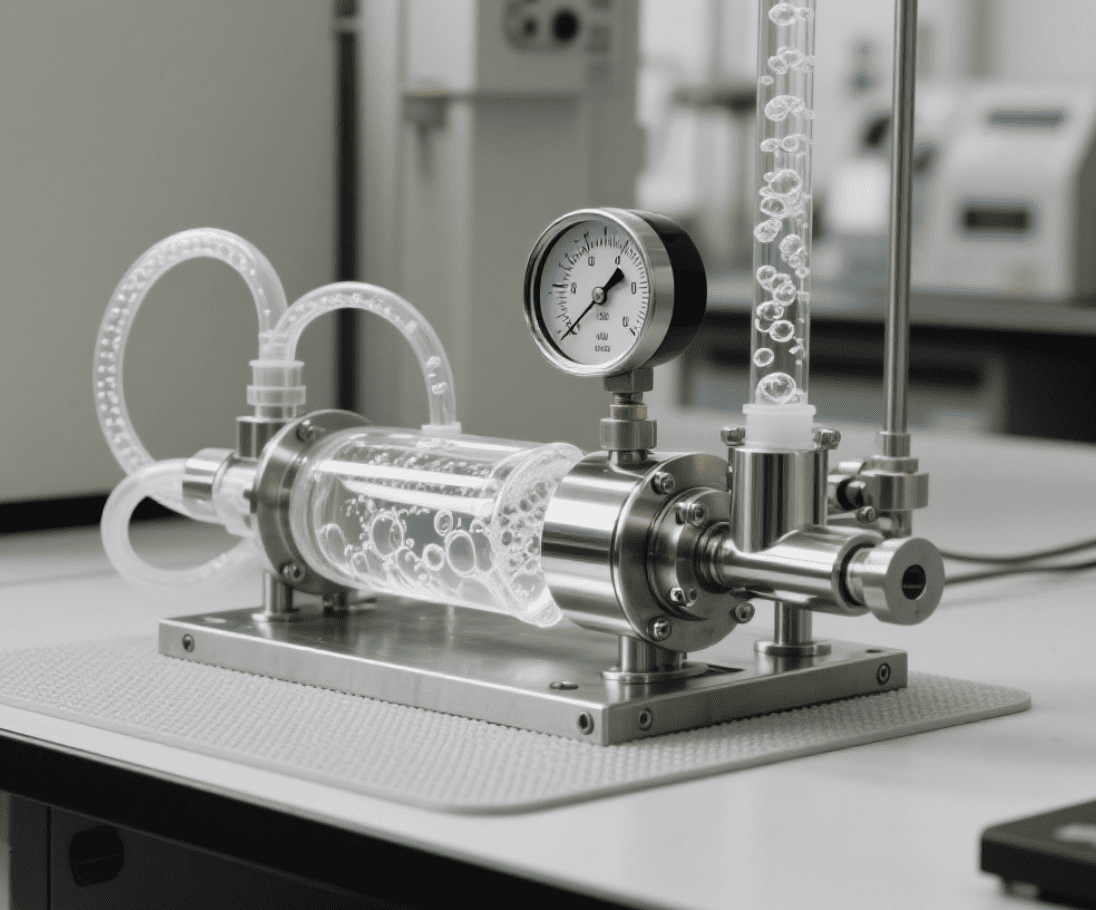
The Process of Carbonating Drinks
The actual process of carbonating drinks is straightforward but requires precision to achieve the right amount of fizz. In industrial settings, carbonation is achieved by pressurizing CO2 into a liquid under controlled conditions:
Pressurization: The liquid is placed in a sealed chamber where CO2 is introduced at high pressure. The pressure forces the CO2 to dissolve into the liquid.
Chilling: The liquid is chilled to a lower temperature to allow more CO2 to dissolve. The colder the liquid, the more gas it can hold.
Sealing: Once the CO2 is dissolved, the liquid is sealed in bottles or cans to maintain the pressure until it's ready to be consumed.
At home, carbonation is done using carbonation machines, which also pressurize CO2 into water. These machines typically use small CO2 canisters that can be adjusted to produce the desired level of fizz. Many people enjoy using these machines because they offer a customizable experience—whether you prefer light carbonation or a more intense fizz.
The Benefits of Carbonated Drinks
While carbonation is mainly associated with taste and texture, it also offers a few health and practical benefits:
Aiding Digestion
Interestingly, carbonated drinks can aid in digestion. The bubbles in these drinks can promote burping, which helps relieve bloating and discomfort. Carbonated water, in particular, has been shown to stimulate the production of gastric juices, which can improve digestion and help move food through the digestive system.
Preserving Beverages
Carbonation also acts as a preservative. The high acidity of carbonated drinks inhibits the growth of harmful bacteria, which can extend the shelf life of beverages. This is one reason why carbonated drinks often stay fresh for longer than still beverages.
Frequently Asked Questions
Does carbonation make drinks more acidic?
Yes, carbonation increases the acidity of a drink due to the formation of carbonic acid. However, this acid is relatively mild and doesn’t drastically alter the taste of the drink.
Why do some carbonated drinks go flat faster than others?
Several factors affect how quickly a carbonated drink goes flat, including the amount of CO2 in the liquid, temperature, and storage. Drinks stored in warmer conditions will lose their fizz faster, as CO2 is less soluble in higher temperatures.
Can you carbonate any beverage at home?
While it is possible to carbonate most beverages at home, carbonating water or lighter liquids produces the best results. Thicker liquids like juice or milk can be more challenging to carbonate and may not produce as much fizz.
Conclusion
The science of carbonation is a key element in what makes fizzy drinks so enjoyable. By understanding the process behind carbonation, from the chemistry of CO2 dissolving into liquid to the impact it has on flavor and texture, we can better appreciate why carbonated drinks are so refreshing. Whether you’re drinking soda, sparkling water, or brewing your own fizzy beverages at home, the science of carbonation ensures that each sip delivers the perfect amount of effervescence. So, the next time you crack open a bottle of your favorite fizzy drink, take a moment to appreciate the chemistry that makes it all possible!
 Innovative Features of Modern
Innovative Features of Modern
 The Science Behind Carbonated
The Science Behind Carbonated
 How to Care for Carbonated Sod
How to Care for Carbonated Sod
 The Evolution of Carbonated So
The Evolution of Carbonated So